Towards reliable ab initio sublimation pressures for organic molecular crystals – are we there yet?†
Abstract
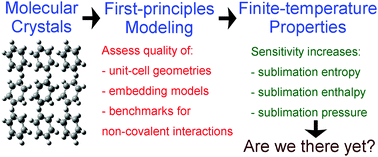
Knowledge of molecular crystal sublimation equilibrium data is vital in many industrial processes, but this data can be difficult to measure experimentally for low-volatility species. Theoretical prediction of sublimation pressures could provide a useful supplement to experiment, but the exponential temperature dependence of sublimation (or any saturated vapor) pressure curve makes this challenging. An uncertainty of only a few percent in the sublimation enthalpy or entropy can propagate to an error in the sublimation pressure exceeding several orders of magnitude for a given temperature interval. Despite this fundamental difficulty, this paper performs some of the first ab initio predictions of sublimation pressure curves. Four simple molecular crystals (ethane, methanol, benzene, and imidazole) have been selected for a case study showing the currently achievable accuracy of quantum chemistry calculations. Fragment-based ab initio techniques and the quasi-harmonic approximation are used for calculations of cohesive and phonon properties of the crystals, while the vapor phase is treated by the ideal gas model. Ab initio sublimation pressure curves for model compounds are compared against their experimental counterparts. The computational uncertainties are estimated, weak points of the computational methodology are identified, and further improvements are proposed.


 Please wait while we load your content...
Please wait while we load your content...
