Foreign atom encapsulated Au12 golden cages for catalysis of CO oxidation†
Abstract
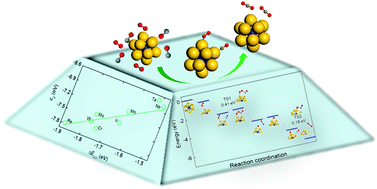
Gold clusters are known for their unique catalytic properties, among which, endohedral gold clusters doped with heteroatoms have remarkable stabilities, with electronic structures tunable by both cluster size and doping element. Thus, it is intriguing and imperative to understand the principles for modulating the catalytic behaviors of these novel clusters. Here, we exploit experimentally produced M@Au12 (M = transition metal) cage clusters for catalysis of CO oxidation. The doping effects of 3d, 4d and 5d transition metals (V, Cr, Mn, Nb, Mo, Ta, W and Re) on the catalytic properties were systematically explored by first-principles calculations. Among the considered M@Au12 clusters, Cr@Au12 and Mn@Au12 provide a suitable binding strength with reaction intermediates and are highly active for CO oxidation with reaction barriers of 0.41 eV under the Langmuir–Hinshelwood mechanism. More importantly, we establish a distinct relationship between catalytic activity and the M–Au bond order and the d orbital center of the M@Au12 clusters, which would help tailor their catalytic performance with atomistic precision and enable utilization of these stable gold cages for catalysis of various chemical processes.


 Please wait while we load your content...
Please wait while we load your content...