Structural interpretation of the 31P NMR chemical shifts in thiophosphate and phosphate: key effects due to spin–orbit and explicit solvent†
Abstract
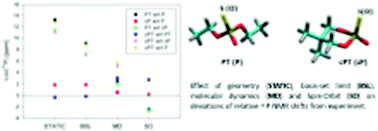
Structural interpretation of the 31P NMR shifts measured in O,O-diethyl thiophosphate (PT), 5,5-dimethyl-2-mercapto-1,3,2-dioxaphosphorinane 2-oxide (cPT), diethylphosphate (P) and 5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphinane 2-oxide (cP) was obtained by means of theoretical calculations including the effects of geometry, molecular dynamics, and solvent, relativistic effects and the effect of NMR reference. NMR calculations employed the B3LYP, BP86, BPW91, M06-2X, PBE0, MP2, and HF methods, the Iglo-n (n = II, III), cc-pVnZ (n = D, T, Q, 5), and pcS-n (n = 0, 1, 2, 3, 4) Gaussian-type basis sets and the Slater-type QZ4P atomic basis. Water solvent was described explicitly and/or implicitly. The effects due to molecular dynamics were calculated using molecular dynamics simulations with the GAFF force field and the TIP3P water molecules, and alternatively by means of the zero-point ro-vibrational averaging. Relativistic effects included the spin–orbit calculated within the two-component zero-order relativistic approximation and the effect with the four-component DFT method. Optimal geometries and large-amplitude dynamical motions within the “opened” PT and P molecules contrasted with notably different geometries and confined dynamical motions within the cPT and cP “closed” molecules. These structure-dynamical differences together with the different chemical structures of thiophosphate and phosphate due to a non-esterified sulphur or oxygen atom within the group considerably affected the magnitudes of 31P NMR shifts. The theoretical calculations enabled accurate and reliable structure-dynamical interpretation of the measured 31P NMR shifts. The effects due to explicit solvent and relativity turned out to be indispensable for obtaining accurate 31P NMR shifts particularly in the thiophosphates. Replacement of the non-esterified oxygen atom in the phosphate with sulphur makes NMR shielding of the phosphorus atom qualitatively different as compared to the NMR shielding of the phosphorus atom in phosphate, H3PO4 and PH3.


 Please wait while we load your content...
Please wait while we load your content...