Contribution of substrate reorganization energies of electron transfer to laccase activity†
Abstract
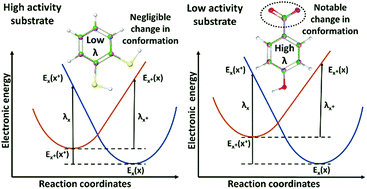
Electron transfer is the most fundamental reaction in chemistry, yet its exact mechanistic details are often complex. Laccases are important electron-transfer enzymes of substantial utility in bleaching, bioremediation, catalytic synthesis, and enzymatic fuel cells. These multi-copper oxidases catalyze the one-electron oxidation of substrates by outer-sphere electron transfer to a copper T1 site, and subsequent intramolecular electron transfer to a tri-nuclear copper site where O2 is reduced to water. Understanding the molecular mechanism of the first, supposedly rate-determining pure electron transfer step is of major fundamental and technological interest. It is widely thought that the difference in the half potentials of the substrate and the T1 copper enables the powerful electron abstraction from nearby substrates. However, the reorganization energy during electron transfer could also contribute to catalytic turnover. To explore this, we computed the self-exchange reorganization energies of 54 substrates with experimentally known activity or kcat data using density functional theory. We show that the energy costs of changing the substrate geometries during electron removal correlate significantly with experimental activity data with a physically meaningful direction of correlation. This means that substrate electronic reorganization, rather than only potential differences, plays a role in the activity of electron transfer proteins such as laccases. This finding is consistent with the Marcus theory and suggests that the first electron transfer step from substrate to T1 is rate-determining in the real enzymes; the electronic reorganization energies can rationalize “good” vs. “bad” laccase substrates, which has not previously been possible.


 Please wait while we load your content...
Please wait while we load your content...