Dissociative electron attachment to HNO3 and its hydrates: energy-selective electron-induced chemistry
Abstract
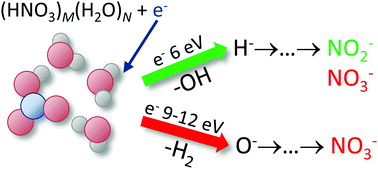
We probe the negative ion production upon the interaction of free electrons with gas-phase HNO3 and its mixed clusters with water. The electron-induced chemistry changes strongly with clustering, exhibiting significant electron energy dependence. For HNO3 hydrates, we identified three involved energy ranges with different behavior: low energies up to about 3.5 eV, an intermediate energy range around 6 eV, and a high energy range, approximately above 9 eV. The major difference is the degree to which the major gas-phase product, NO2−, is converted to NO3−. The latter is the dominant stratospheric anion. Its appearance due to the electron interaction with mixed HNO3/water ice particles thus strongly depends on the electron energy. We discuss the elementary processes and reaction pathways behind the anion conversion.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...