The impact of carbonate solvents on the self-discharge, thermal stability and performance retention of high voltage electrochemical double layer capacitors†
Abstract
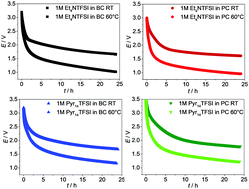
Advanced electrolytes for supercapacitors with high electrochemical stability are necessary to improve the suitability of supercapacitors for many applications. In this work we investigated electrolytes based on the solvent propylene carbonate (PC) and butylene carbonate (BC). A comparison of different solvent–salt combinations shows that 1 M N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (Pyr14TFSI) in BC is superior to conventionally used PC-based electrolytes examined in this work in terms of voltage window and capacitance. In order to gain a better understanding of the influence of the ions and the solvent on the formation of the electrochemical double layer, the self-discharge mechanism and its temperature dependence have been investigated in detail. By coupling thermogravimetry (TGA), infrared spectroscopy (IR), gas chromatography and mass spectrometry (GC-MS), also decomposition and temperature stability have been assessed.
- This article is part of the themed collection: Bunsentagung 2019: Functional Materials


 Please wait while we load your content...
Please wait while we load your content...