Which types of clay minerals fix cesium ions effectively? the “cavity-charge matching effect”†
Abstract
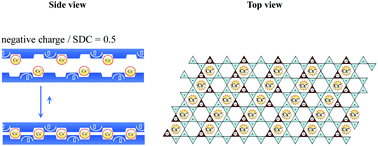
How can radioactive Cs+ ions be removed from aqueous solution? From this perspective, the adsorption of Cs+ was investigated by using five types of clay minerals possessing different charge exchange capacities. The fixation ability for Cs+ depended on the charge exchange capacity of the clay minerals. Phlogopite and vermiculite, where the number of charges is almost equal to half the number of siloxane ditrigonal cavities in the structure, exhibited a strong Cs+ fixation ability among these clay minerals. In these clay minerals, effective interlayer collapse, which leads to quasi-irreversible adsorption of Cs+, is expected from the introduction of Cs+ into the layer space. This is named the “cavity-charge matching effect”. This study clarifies why only phlogopite and vermiculite can fix Cs+ quite strongly among various types of clay minerals. These findings are beneficial for removing radioactive Cs+ ions from the environment using clay minerals through the cavity-charge matching effect.


 Please wait while we load your content...
Please wait while we load your content...