Effect of extending conjugation via thiophene-based oligomers on the excited state electron transfer rates to ZnO nanocrystals
Abstract
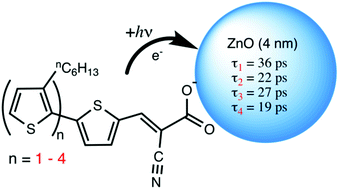
Oligothiophene dyes with two to five thiophene units were anchored to oleate-capped, quantum-confined zinc oxide nanocrystals (ZnO NCs) through a cyanoacrylate functional group. While the fluorescence of the bithiophene derivative was too weak for meaningful quenching studies, the fluorescence of the dyes with three, four and five thiophene rings was quenched upon binding to the NCs. Ultrafast pump–probe spectroscopy was used to observe the singlet excited states of the free dyes dissolved in dichloromethane as well as attached to a ZnO NC dispersed in the same solvent. When the dyes were bound to ZnO NCs, ultrafast spectroscopic measurements revealed rapid disappearance of the singlet excited state and appearance of a new transient absorption at higher energy that was assigned to the oxidized dye based on the similar absorption observed upon oxidation of the dye using nitrosonium ion. The appearance lifetimes of the oxidized dyes were assigned to the excited state electron transfer and were 36 ± 2, 22.3 ± 3.9, 26.5 ± 1.5 and 19.4 ± 0.8 ps for bi-, ter-, quarter- and quinquethiophene dyes respectively. Two factors contributed to the similarity in the electron transfer lifetime. First the excited state energies of the dyes were similar, and second, the free energy for electron transfer reaction was sufficiently large to move the event into the energy-independent regime.


 Please wait while we load your content...
Please wait while we load your content...
