Excited-state dynamics of heteroleptic copper(i) photosensitizers and their electrochemically reduced forms containing a dipyridophenazine moiety – a spectroelectrochemical transient absorption study†
Abstract
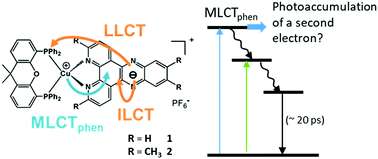
The electrochemically singly-reduced Cu(I) photosensitizers of the type [Cu(xant)(N^N)]+ (with xant = xantphos ligand and N^N = bidentate diimine ligand: dipyrido[3,2-a:2′,3′-c]phenazine = dppz or 3,6,11,12-tetramethyl-dipyrido[3,2-a:2′,3′-c]phenazine = tmdppz) exhibit a metal-to-ligand charge transfer (MLCT) transition from the Cu(I) center to the reduced dppz˙− ligand. This special behavior makes them promising candidates for two-electron accumulation. Consequently, the photoinduced excited-state processes of [Cu(xant)(dppz)]+ (1) and [Cu(xant)(tmdppz)]+ (2) were investigated in solution by femtosecond transient absorption spectroelectrochemistry. Furthermore, the influence of the methyl substitution at the dppz ligand on the transient dynamics was revealed. Moreover, both singly-reduced species 1− and 2− possess short-lived excited states (10–20 ps) when excited into the MLCTphen or the low-lying states, representing an obstacle for the possible two-electron photoaccumulation.


 Please wait while we load your content...
Please wait while we load your content...