Oxygen surface exchange and diffusion in Pr1.75Sr0.25Ni0.75Co0.25O4±δ
Abstract
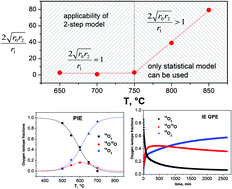
Oxygen surface exchange and diffusion in Pr1.75Sr0.25Ni0.75Co0.25O4±δ have been investigated using two methods: pulsed isotope exchange (PIE) and oxygen isotope exchange with gas phase equilibration (IE GPE). Oxygen surface exchange kinetics is considered in the framework of two-step models including two consecutive stages: dissociative adsorption of oxygen and incorporation of oxygen adatoms into the crystal lattice. The rates of oxygen heterogeneous exchange (rH) as well as the rates of dissociative adsorption (ra) and oxygen incorporation (ri) have been calculated. The applicability of the two-step model is discussed based on the concept of a novel two-step mechanism with distributed rates of dissociative adsorption and incorporation of oxygen. It is shown that the two-step model can be applicable for the description of oxygen exchange kinetics in Pr1.75Sr0.25Ni0.75Co0.25O4±δ only at temperatures below 750 °C. Above this temperature, only the statistical model with distributed rates can be used. At low temperatures (<750 °C), the oxygen incorporation rate is found to be smaller than the rate of oxygen dissociative adsorption. Thus, under these experimental conditions the stage of oxygen incorporation is considered to be rate-determining. When increasing the temperature, the difference between ra and ri decreases and the stages become competing. The oxygen isotope exchange kinetic profiles obtained using the IE GPE method are found to be complicated and include a surface exchange stage as well as at least two diffusion relaxation processes. The reasons for the existence of these two processes are discussed.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...