Geometry and energetics of CO adsorption on hydroxylated UiO-66
Abstract
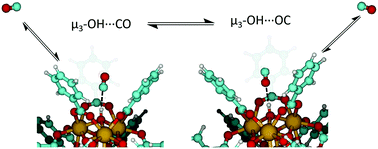
The metal–organic framework (MOF), UiO-66, contains Brønsted acidic and Lewis acidic sites that play an important role in sorption, separation, and potential catalytic processes involving small gaseous molecules. As such, studies into the sequestration and separation of CO within UiO-66 provides a fundamental understanding of small gas molecule adsorption within a highly porous, tunable and environmentally stable MOF. Infrared spectroscopic measurements, in combination with density functional theory, were employed to characterize the binding energetics between bridging hydroxyl groups at MOF nodes and the adsorbate, CO. Two unique binding configurations between CO and the μ3-OH groups were identified based on differing stretching vibrations of COads when interacting through the C- and O-atom of the molecule. Variable temperature infrared spectroscopy (VTIR) was employed to attain energetics of CO adsorption (−17 kJ mol−1) and isomerization from the carbonyl to the isocarbonyl configuration (4 kJ mol−1). Results suggest that CO–hydroxyl interactions, while weak in nature, play a critical role in CO adsorption within the confined pore environment of UiO-66.


 Please wait while we load your content...
Please wait while we load your content...
