Electronic structure and reactivity of Fe(iv)oxo species in metal–organic frameworks
Abstract
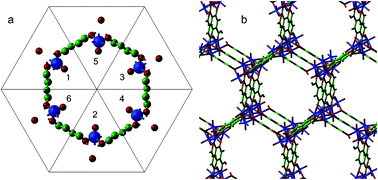
We investigate the potential use of Fe(IV)oxo species supported on a metal–organic framework in the catalytic hydroxylation of methane to produce methanol. We use periodic density-functional theory calculations at the 6-31G**/B3LYP level of theory to study the electronic structure and chemical reactivity in the hydrogen abstraction reaction from methane in the presence of Fe(IV)O(oxo) supported on MOF-74. Our results indicate that the Fe(IV)O moiety in MOF-74 is characterised by a highly reactive (quintet) ground-state, with a distance between Fe(IV) and O(oxo) of 1.601 Å, consistent with other high-spin Fe(IV)O inorganic complexes in the gas phase and in aqueous solution. Similar to the latter systems, the highly electrophilic character (and thus the reactivity) of Fe(IV)O in MOF-74 is determined by the presence of a low-lying anti-bonding virtual orbital (3σ*), which acts as an electron acceptor in the early stages of the hydrogen atom abstraction from methane. We estimate an energy barrier for hydrogen abstraction of 50.77 kJ mol−1, which is comparable to the values estimated in other gas-phase and hydrated Fe(IV)O-based complexes with the ability to oxidise methane. Our findings therefore suggest that metal–organic frameworks can provide suitable supports to develop new solid-state catalysts for organic oxidation reactions.


 Please wait while we load your content...
Please wait while we load your content...