Ultrafast internal conversion dynamics of bilirubin bound to UnaG and its N57A mutant†
Abstract
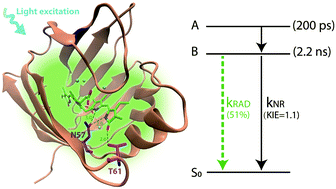
Fluorescent proteins (FPs) have become fundamental tools for live cell imaging. Most FPs currently used are members of the green fluorescent protein super-family, but new fluorophores such as bilin-FPs are being developed and optimized. In particular, the UnaG FP incorporates bilirubin (BR) as a chromophore, enhancing its fluorescence quantum yield by three orders of magnitude relative to that in solution. To investigate the mechanism of this dramatic enhancement and provide a basis for further engineering of UnaG and other tetrapyrrole-based fluorophores, we performed picosecond fluorescence and femtosecond transient absorption measurements of BR bound to UnaG and its N57A site-directed mutant. The dynamics of wt-UnaG, which has a fluorescence QY of 0.51, are largely homogeneous, showing an excited state relaxation of ∼200 ps, and a 2.2 ns excited-state lifetime decay with a kinetic isotope effect (KIE) of 1.1 for D2O vs. H2O buffer. In contrast, for UnaG N57A (fluorescence QY 0.01) the results show a large spectral inhomogeneity with excited state decay timescales of 47 and 200 ps and a KIE of 1.4. The non-radiative deactivation of the excited state is limited by proton transfer. The loss of direct hydrogen bonds to the endo-vinyl dipyrrinone moiety of BR leads to high flexibility and structural heterogeneity of UnaG N57A, as seen in the X-ray crystal structure.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
