A study on the comprehension of differences in specific kinetic energy of TKX-50 and HMX from the perspective of gas products
Abstract
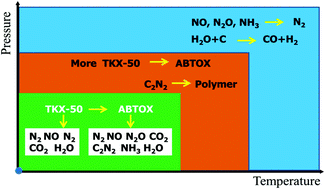
5,5′-Bitetrazole-1,1′-dioxydihydroxylamine salt (TKX-50), a high-energy energetic material, possesses good safety and energy properties. The energy characteristic data of TKX-50 are commonly generated via theoretical simulation and experimental measurements. Interestingly, the detonation velocity of TKX-50 is higher than HMX, but the specific kinetic energy of TKX-50 is the opposite. Thus, a systematic study on the decomposition mechanism of TKX-50 is important to establish the reasons for this variation in specific kinetic energy. Although the thermal decomposition mechanism of TKX-50 has been reported, the specific compositional changes of its gas products under different heating conditions remain unknown, hindering a comprehensive understanding of the mechanism from the perspective of gas products. Herein, the gas products of TKX-50 and HMX in thermal decomposition and thermal explosion are investigated and compared. It was found that more TKX-50 is converted to ABTOX for further decomposition when the heating rate increases. ABTOX can decompose to C2N2, which is prone to polymerization, generating a solid residue under high temperature and pressure. Although polymerized C2N2 decomposes and burns during the explosion, it delays the time of TKX-50 reaching its maximum amount of outgassing, thereby affecting its specific kinetic energy. Furthermore, in the thermal explosion, compared with HMX, TKX-50 generates less H2 and CO. Since the combustion heat of hydrogen is much higher than that of carbon, the more hydrogen generated, the higher the detonation heat obtained. Therefore, TKX-50 has a lower detonation heat, which also affects its specific kinetic energy.


 Please wait while we load your content...
Please wait while we load your content...