Understanding the charging dynamics of an ionic liquid electric double layer capacitor via molecular dynamics simulations†
Abstract
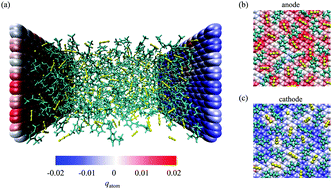
We investigate the charging phenomena of an electric double layer capacitor (EDLC) by conducting both equilibrium and non-equilibrium molecular dynamics (MD) simulations. A graphene electrode and 1-ethyl-3-methylimidazolium thiocyanate ([EMIM]+[SCN]−) ionic liquid were used as a system for the EDLC. We clarify the ionic layer structure and show that an abrupt change of the ionic layers leads to a high differential capacitance of the EDLC. The charging simulations reveal that the charging dynamics of the EDLC is highly dependent on the rearrangement of the ionic layer structure. Particularly, the electrode charge during the charging process is consistent with the perpendicular displacement of ionic liquid molecules. From this property, we analyze the contribution of each molecular ion to the electrode charge stored during charging. Charging of the EDLC is largely dependent on the desorption of the co-ions from the electrode rather than the adsorption of the counter-ions. In addition, the contribution of bulk ions to the charge stored in the EDLC is as important as that of ions adjacent to the electrode surface contrary to the conventional viewpoint. From these results, we identify the charging mechanism of the EDLC and discuss the relevance to experimental results. Our findings in the present study are expected to play an important role in designing an efficient EDLC with a novel perspective on the charging of the EDLC.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...