Temperature dependence of the ultrafast vibrational echo spectroscopy of OD modes in liquid water from first principles simulations
Abstract
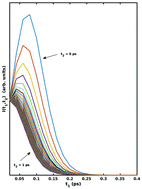
The rate of vibrational spectral diffusion of OD/OH stretch modes of water is known to be interconnected with the hydrogen bond rearrangement dynamics in aqueous media as found in several recent experiments and molecular simulations. In the present study, the temperature dependence of vibrational spectral diffusion of OD stretch modes in liquid water is investigated from first principles by using the method of ab initio molecular dynamics. Kinetic rates obtained from the frequency time correlation function (FTCF), the slope of the 3-pulse photon echo (S3PE) and local structure correlation functions are used in the Arrhenius equation to determine the energy barrier for hydrogen bond rearrangement in liquid water. The slope of the 3-pulse photon echo is determined within the cumulant and Condon approximations. Although the trend found at the low temperature is slightly non-Arrhenius, the barrier for hydrogen bond rearrangement is found to be around 4.2 kcal mol−1 from all the metrics considered here. It is in good agreement with the hydrogen bond energy determined from different experiments and theoretical studies. Based on the findings, a strong correlation between the vibrational spectral diffusion timescale and hydrogen bond dynamics is identified, which gets even more pronounced at low temperatures. Spatio-temporal correlations between frequency fluctuations and the local hydrogen bond network are also explored.


 Please wait while we load your content...
Please wait while we load your content...