Theoretical modelling of the dynamics of primary photoprocess of cyclopropanone
Abstract
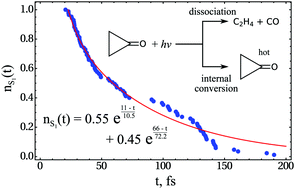
Photodecomposition of cyclopropanone is investigated by static quantum chemical calculations and non-adiabatic molecular dynamics (NAMD) simulations. The quantum chemical calculations are carried out by an ensemble density functional theory (eDFT) method capable of delivering high quality results for the ground and excited electronic states of molecules with dissociating bonds. In the NAMD simulations, this method is combined with a novel trajectory surface hopping (TSH) methodology derived from the exact factorization of the electronic-nuclear wavefunction. An ultrafast biexponential decay of the S1 state of cyclopropanone is predicted, where the short (ca. 30 fs) decay time is due to the trajectories reaching the conical intersection (CI) seam on the first approach and the long (ca. 120 fs) decay time is due to recrossing of the CI seam. The experimentally observed dependence of the dissociation (C3H4O* → C2H4 + CO) quantum yield on the excitation wavelength is correctly reproduced by the NAMD simulations. The dependence is explained by the necessity to excite certain vibrational normal modes (e.g., a ring stretching mode with the frequency of 769 cm−1) to break a lateral C–C bond remaining intact at the geometries of the CI seam.


 Please wait while we load your content...
Please wait while we load your content...