Solvation structure of poly-m-phenyleneisophthalamide (PMIA) in ionic liquids†
Abstract
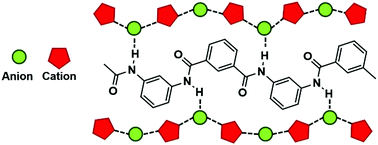
Polyaramids are a class of high-performance polymers, known for their high mechanical strength and chemical and thermal stability. Their ability to create a network of intermolecular hydrogen bonds causes them to be very poorly soluble in conventional solvents. Hazardous solvents such as N-methylpyrrolidone (NMP) and dimethylacetamide (DMA), in combination with an inorganic salt such as CaCl2, are currently used for the synthesis and processing of polyaramids. Ionic liquids are proposed as suitable greener alternatives. In this work, we studied the solubility and dissolution mechanism of the meta-oriented polyaramid poly-m-phenyleneisophthalamide (PMIA) in a wide range of ionic liquids. It was found that, similarly to cellulose, PMIA could be dissolved readily and in large amounts in ionic liquids containing a strongly coordinating anion (such as chloride, acetate and dialkylphosphate) and an imidazolium cation. Hydrogen bonding between the anion and the amide NH of PMIA is the main solvent–solute interaction. An odd–even effect in solubility occurred when altering the length of the side chains on the imidazolium cation. Furthermore, it was found that the presence of hydrogen bond donating CH moieties on the cation is a necessary condition for dissolution. The exact role of these hydrogen bond donors was investigated by FTIR and 13C NMR spectroscopy. It was found that there is no significant interaction between the hydrogen atoms of the imidazolium ring and the amide carbonyl groups. Rather, the hydrogen bond donors are needed to stabilize the solvation shell around PMIA through alternating cation–anion interactions.


 Please wait while we load your content...
Please wait while we load your content...