Unprecedented O:⇔:O compression and H↔H fragilization in Lewis solutions
Abstract
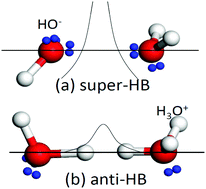
Charge injection in terms of lone pairs ‘:’, protons, and ions upon acid and base solvation mediates the hydrogen bonding network and properties of Lewis solutions, and is ubiquitously important in many subject areas of Chemical Physics. This work features the recent progress and future trends in this aspect with a focus on the solute–solvent interactions and hydrogen bond (O:H–O or HB) transition from the vibration mode of ordinary water to the hydrating states. A combination of the O:H–O bond cooperativity notion, differential phonon spectrometrics, calorimetric detection, and quantum computations clarified the solute capabilities of O:H–O bond transition in HX and YOH (X = Cl, Br, I and Y = Li, Na, K) solutions. The H+ and the lone pair do not stay alone to move or shuttle freely between adjacent H2O molecules, but they are attached to a H2O molecule to form (H3O+ and OH−)·4H2O tetrahedral motifs, which transits an O:H–O bond into the H↔H anti-HB point breaker in acidic solutions and into the O:⇔:O super-HB compressor and polarizer in basic solutions, respectively. H↔H disrupts the solvent network and surface stress, having the same effect of liquid heating on HB bond relaxation and thermal fluctuation on surface stress. The O:⇔:O compression lengthens and weakens the solute H–O bond, which heats up the solution during solvation. The H–O bonds due to H3O+ contract by 3% and due to OH− shrink by 10%. The Y+ and X− ions perform in the same manner as they do in salt solutions to form hydration shells through electrostatic polarization and hydrating H2O dipolar screen shielding. Focusing more on the O:H–O bond transition would be even more promising and revealing than on the manner and mobility of lone pair and proton transportation.


 Please wait while we load your content...
Please wait while we load your content...