Small substituent groups as geometric controllers for tridentate platinum(ii) complexes to effectively suppress non-radiative decay processes
Abstract
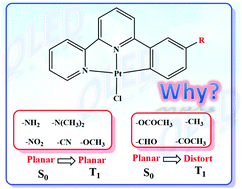
For phosphorescent emitters, the rigidity of the geometry is a crucial indicator, which can directly determine the non-radiative decay rate. In this article, density functional theory (DFT) calculations were performed to investigate the influence of the small substituent groups on the rigidities of tridentate Pt(II) complexes in detail. The calculated results indicate that the small substituent groups can serve as geometric controllers to suppress the structural distortion on going from the ground state (S0) to the lowest-lying triplet excited state (T1) (Jahn–Teller distortion). For instance, when electron-donating substituent groups, including –NH2, –N(CH3)2 and –OCH3, were employed, the rigidities of the corresponding Pt(II) complexes can be effectively enhanced because the highest occupied molecular orbital (HOMO)–HOMO−1 energy gaps could be increased. Different from the electron-donating substituent groups, electron-withdrawing substituent groups, i.e., –NO2 and –COCH3, can cause a negligible change in HOMO and HOMO−1 energies during the S0 → T1 transition process, and therefore, for Pt-NO2 and Pt-COCH3, no Jahn–Teller distortion occurs. According to the calculated results, the rigidities of tridentate Pt(II) complexes could be raised via tuning the energies of the frontier molecular orbital (FMO) with the help of small substituent groups.


 Please wait while we load your content...
Please wait while we load your content...