Distance-dependent formation of electronic charge-transfer states in the ground states of anthracene and pyrene covalently linked to a TEMPO free radical†
Abstract
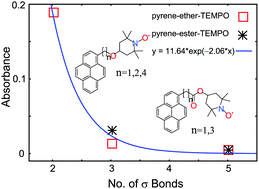
Charge-transfer (CT) electronic states are generally seen in molecules involving interactions between species of low ionization potential and high electron affinity. In this context, the 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) free radical is not considered to be a typical molecule to form charge transfer states with aromatic hydrocarbons. Nevertheless, involvement of such CT states has been invoked in rationalising the spin-dependent photophysical quenching of excited states of aromatic systems by TEMPO during bimolecular collisions. Direct observation of such CT states, however, has been elusive until recently, with our first report on the observation of CT states involving naphthalene and TEMPO moieties covalently linked through a spacer group (Rane et al., J. Fluoresc., 2015, 25, 1351–1361). With a view to demonstrating more systems of CT states involving a TEMPO donor, and establishing a possible dependence on its distance from an acceptor chromophore, we have now extended our investigation to anthracene (An) and pyrene (Py) moieties linked to TEMPO, using two different spacer groups of different lengths. The molecules are An–CH2–O–TEMPO, Py–CH2–O–TEMPO, Py–(CH2)2–O–TEMPO, Py–(CH2)4–O–TEMPO, Py–CH2–CO–O–TEMPO and Py–(CH2)3–CO–O–TEMPO, where a linear alkyl chain containing an ether or an ester moiety constitutes the spacer group. We established the formation of CT states in their ground states by comparing their electronic absorption spectra, steady-state fluorescence spectra and time-resolved fluorescence signals with those of the parent molecules An–CH2–OH, Py–CH2–OH and Py–CH2–COOH. CT bands of appreciable intensity were seen only with An–CH2–O–TEMPO, Py–CH2–O–TEMPO and Py–CH2–CO–O–TEMPO, the molecules with the shortest spacer group. Approximate shapes of the absorption and emission bands of the CT states have been determined. For the rest, very weak bands were seen. Similar trends were seen in their fluorescence lifetimes also. Absorption intensities of the CT bands were found to decrease exponentially with the length of the spacer group. The presence of the ether or the ester moiety in the spacer groups showed little influence on the intensities of the CT bands. Our results are probably the first experimental demonstration of the expected exponential dependence of the efficiency of the formation of CT states on the length of the spacer groups of chromophore–TEMPO linked molecules.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...