Excited-state solvation structure of transition metal complexes from molecular dynamics simulations and assessment of partial atomic charge methods†
Abstract
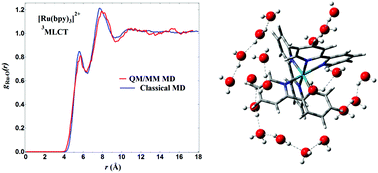
In this work, we investigate the excited-state solute and solvation structure of [Ru(bpy)3]2+, [Fe(bpy)3]2+, [Fe(bmip)2]2+ and [Cu(phen)2]+ (bpy = 2,2′-bipyridine; bmip = 2,6-bis(3-methyl-imidazole-1-ylidine)-pyridine; phen = 1,10-phenanthroline) transition metal complexes (TMCs) in terms of solute–solvent radial distribution functions (RDFs) and evaluate the performance of some of the most popular partial atomic charge (PAC) methods for obtaining these RDFs by molecular dynamics (MD) simulations. To this end, we compare classical MD of a frozen solute in water and acetonitrile (ACN) with quantum mechanics/molecular mechanics Born–Oppenheimer molecular dynamics (QM/MM BOMD) simulations. The calculated RDFs show that the choice of a suitable PAC method is dependent on the coordination number of the metal, denticity of the ligands, and type of solvent. It is found that this selection is less sensitive for water than ACN. Furthermore, a careful choice of the PAC method should be considered for TMCs that exhibit a free direct coordination site, such as [Cu(phen)2]+. The results of this work show that fast classical MD simulations with ChelpG/RESP or CM5 PACs can produce RDFs close to those obtained by QM/MM MD and thus, provide reliable solvation structures of TMCs to be used, e.g. in the analysis of scattering data.


 Please wait while we load your content...
Please wait while we load your content...