Gas-phase synthetic pathways to benzene and benzonitrile: a combined microwave and thermochemical investigation
Abstract
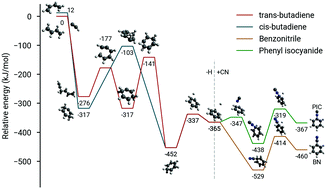
The recent astronomical detection of benzonitrile (C6H5CN) in the cold, starless cloud TMC-1 demonstrates that aromatic chemistry is efficient even in the primordial stages of star and planet formation. C6H5CN may serve as a convenient observational proxy for benzene, which is otherwise challenging to detect in space, provided the chemistry linking these two molecules is tightly constrained. Here we present a high-resolution microwave spectroscopic study in combination with an accurate thermochemical treatment of the formation chemistry of C6H5CN and benzene. We demonstrate that C6H5CN is a highly useful tracer for benzene in the presence of CN radical, either in space or in the laboratory, and by inference, that the reaction C2H + CH2(CH)2CH2 yields benzene, along with its high-energy polar isomer fulvene. In addition, we find that the higher energy isomer, C6H5NC, is formed at <0.1% abundance relative to C6H5CN. By exploiting –CN tagging, formation pathways to produce benzene using a variety of acyclic hydrocarbon precursors are then explored. A robust, self-consistent, and chemically accurate theoretical treatment has also been undertaken for several key reactions. The results are discussed both in the context of aromatic molecule synthesis and astrochemistry.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
