Nanoporous gold functionalized with praseodymia–titania mixed oxides as a stable catalyst for the water–gas shift reaction†
Abstract
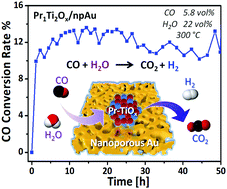
Dealloyed nanoporous metals hold great promise in the field of heterogeneous catalysis; however their tendency to coarsen at elevated temperatures or under catalytic reaction conditions sometimes limit further applications. Here, we report on a highly stable nanoporous gold catalyst (npAu) functionalized with praseodymia–titania mixed oxides as synthesized by a sol–gel method. Specifically, we used aberration-corrected transmission electron microscopy to study the morphology and the interface between the oxide deposits and the npAu substrate at the atomic level. Based on electron energy loss spectroscopy (EELS), it is concluded that Pr–TiOx mixed oxides form a solid solution. Flow reactor tests reveal that the Pr–TiOx functionalized nanoporous gold is not only highly active but also very stable for the water gas shift reaction in a large temperature range (180–400 °C). Our results demonstrate the potential of engineering the compositions of oxides coatings on npAu for advanced functional systems.


 Please wait while we load your content...
Please wait while we load your content...