A vacuum ultraviolet photoionization study on the formation of methanimine (CH2NH) and ethylenediamine (NH2CH2CH2NH2) in low temperature interstellar model ices exposed to ionizing radiation†
Abstract
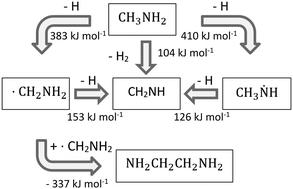
Methylamine (CH3NH2) and methanimine (CH2NH) represent essential building blocks in the formation of amino acids in interstellar and cometary ices. In our study, by exploiting isomer selective detection of the reaction products via photoionization coupled with reflectron time of flight mass spectrometry (Re-TOF-MS), we elucidate the formation of methanimine and ethylenediamine (NH2CH2CH2NH2) in methylamine ices exposed to energetic electrons as a proxy for secondary electrons generated by energetic cosmic rays penetrating interstellar and cometary ices. Interestingly, the two products methanimine and ethylenediamine are isoelectronic to formaldehyde (H2CO) and ethylene glycol (HOCH2CH2OH), respectively. Their formation has been confirmed in interstellar ice analogs consisting of methanol (CH3OH) which is ioselectronic to methylamine. Both oxygen-bearing species formed in methanol have been detected in the interstellar medium (ISM), while for methanimine and ethylenediamine only methanimine has been identified so far. In comparison with the methanol ice products and our experimental findings, we predict that ethylenediamine should be detectable in these astronomical sources, where methylamine and methanimine are present.


 Please wait while we load your content...
Please wait while we load your content...
