Ultrafast excitation energy transfer in a benzimidazole–naphthopyran donor–acceptor dyad†
Abstract
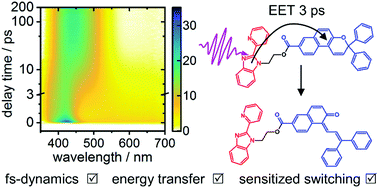
The excited-state dynamics of a donor–acceptor dyad composed of 1-propyl-2-pyridinyl-benzimidazole (PPBI) as donor and the photochromic molecular switch diphenylnaphthopyran (DPNP) as acceptor linked via an ester bridge has been investigated by a combination of static and time-resolved spectroscopies and quantum chemical calculations. The UV absorption spectrum of the dyad is virtually identical to the sum of the spectra of its individual constituents, indicating only weak electronic coupling between the donor and acceptor in the electronic ground state. After selective photoexcitation of the PPBI chromophore in the dyad at λpump = 310 nm, however, a fast electronic energy transfer (EET) from the donor to the acceptor is observed, by which the lifetime of the normally long-lived excited state of PPBI is reduced to a few ps. Enabled by the EET, the acceptor switches from its ring-closed naphtopyran form to its ring-opened merocyanine form. The singular value decomposition-based global analyses of the measured femtosecond time-resolved transient absorption spectra of the dyad and its two building blocks as reference compounds allowed us to determine a value for the EET time constant in the dyad of τ = 2.90 ± 0.60 ps. For comparison, Förster theory predicts characteristic FRET times between 1.2 ps ≤ τ ≤ 4.2 ps, in good agreement with the experimental result.


 Please wait while we load your content...
Please wait while we load your content...