Evaluation of the aggregation process in a mixture of propofol and benzocaine†
Abstract
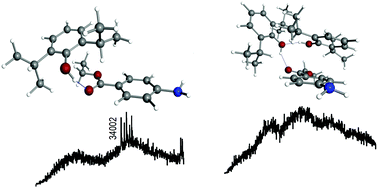
We report on a mass-resolved IR spectrosopic study on propofol–benzocaine aggregates. This is a complex system due to the several conformational isomers that both monomers may adopt and to the combination of functional groups they present, which allow the molecules to interact in many possible ways. However, our results demonstrate that a single conformation is favored for each stoichiometry. In the heterodimer, propofol acts as a proton donor to the ester group of benzocaine, while the whole cluster is stabilized by dispersive forces. These dispersive forces account for an important part of the system's stabilization energy as the calculations suggest. Propofol does not show any affinity for the amino group of benzocaine, even when a second molecule of propofol is introduced. These results demonstrate the difficulty in anticipating the aggregation preferences of even small organic molecules.
- This article is part of the themed collection: Challenges in spectroscopy: accuracy vs interpretation from isolated molecules to condensed phases


 Please wait while we load your content...
Please wait while we load your content...