A quasielastic and inelastic neutron scattering study of the alkaline and alkaline-earth borohydrides LiBH4 and Mg(BH4)2 and the mixture LiBH4 + Mg(BH4)2†
Abstract
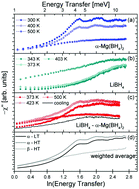
Quasielastic neutron scattering was used to investigate the low energy transfer dynamics of the complex borohydrides Mg(BH4)2 in the α- and β-modifications, LiBH4 in the low and high temperature crystal structure, and an 1 : 1 molar mixture of LiBH4 + α-Mg(BH4)2. All investigated compounds show a rich dynamic behaviour below an energy range of ΔE = 10 meV with the superposition of rotational dynamics of the constituent [BH4]− anions and low lying lattice modes. For Mg(BH4)2, the rotational diffusion of the [BH4] units was found to be much more activated in the metastable β-polymorph compared to the α-phase, and the low lying lattice modes are even softer in the former crystal structure. In Mg(BH4)2, the structural phase transition is mainly governed by the lattice dynamics, while alkaline LiBH4 exhibits a transition of the [BH4] rotations around the phase transition temperature. Ball milled LiBH4 + α-Mg(BH4)2 remains a physical mixture of the parent compounds and each component retains its characteristic dynamic signature up to the melting temperature.


 Please wait while we load your content...
Please wait while we load your content...