Rate constants for collision-induced emission of O2(a1Δg) with He, Ne, Ar, Kr, N2, CO2 and SF6 as collisional partners
Abstract
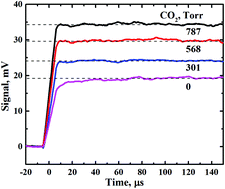
Rate constants for singlet oxygen collision induced emission of the a1Δg–X3Σ−g transition at 1.27 μm were measured for CO2, N2, SF6, and rare gases as collisional partners. Photolysis of ozone by 266 nm laser radiation produced singlet oxygen. We performed direct measurements of pressure dependences of the 1.27 μm emission intensity for partner gases. The measured rate constants kMa–X in the units of 10−24 cm3 s−1 are as follows: CO2 – 10 ± 2; N2 – 3.2 ± 0.6; SF6 – 7 ± 1; He – 1.1 ± 0.3; Ne – 1.3 ± 0.3; Ar – 2.8 ± 0.6; Kr – 6 ± 1. The measured values of kMa–X are close to the values calculated from absorption measurements. Considering the known rate constants kMb–a for the b1Σg+–a1Δg transition in the gas phase we found that the ratio kMa–X/kMb–a was constant and independent of a collisional partner according to the “spin–orbit based” mechanism of intensity borrowing proposed by Minaev (THEOCHEM, 1989, 183, 207). However, this ratio amounted to (1.3 ± 0.2) × 10−4, which is considerably lower than the theoretically predicted value of (3–6) × 10−4.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...