Distinguishing ionic and radical mechanisms of hydroxylamine mediated electrocatalytic alcohol oxidation using NO–H bond dissociation energies†
Abstract
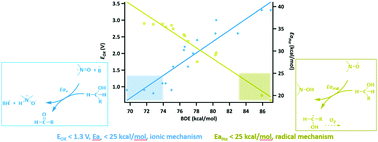
The mechanism of N-oxyl radical catalyzed oxidation is a long-standing scientific problem. In this work, radical or ionic mechanisms in electrocatalytic oxidation of alcohols are discussed on the NO–H bond dissociation energy (BDE) scale. A thermodynamic model was built to outline the range of BDEs for the catalysts that react via the two mechanisms. The N-oxyl radical catalyzed electrocatalytic benzyl alcohol oxidations with NO–H BDEs smaller than 74 kcal mol−1 reacted by an ionic mechanism, and with BDEs greater than 78 kcal mol−1 reacted by a radical mechanism. Oxidizing aliphatic alcohols via a radical mechanism requires catalysts with BDEs even greater than that of N-hydroxyphthalimide (NHPI), and the ionic mechanism requires catalysts with BDEs smaller than 74 kcal mol−1. With either the ionic or radical mechanism, catalysts with larger BDEs correspond to a smaller activation energy of the key step. Future design of N-oxyls with catalytic activity in alcohol oxidation can be streamlined by our efforts.


 Please wait while we load your content...
Please wait while we load your content...