Why does 4-biphenyl carbonyl azide have ultra-short lived excited states? An ultrafast UV-vis spectroscopic and computational study†
Abstract
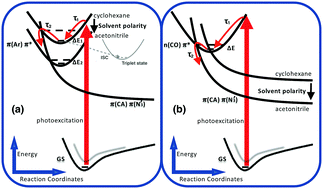
Upon excitation at 308 nm, 4-biphenyl carbonyl azide (4-BpCON3) shows unusually fast decay of transient absorption associated with the first excited singlet state, with time constants of several ps in MeOH, acetonitrile, and CHCl3. In cyclohexane and cyclohexene, the lifetimes are ca. 0.3 ps, which is in stark contrast to the lifetimes of hundreds of ps in the case of 2-naphthoyl azide. Furthermore, photolysis at 266 and 308 nm brought about the same yields of nitrene and isocyanate products. To understand these findings, we also applied ultrafast transient absorption spectroscopy to the structurally related molecule, fluorene-2-carbonyl azide (F2CON3), in which the two phenyl rings are fixed in a plane by a methylene group. Both carbonyl azides (biphenyl and fluorenyl) have very short lived excited states in different solvents, indicating that the twisting of phenyl rings is not the reason for the fast decay. Theoretical studies using time dependent density functional theory (TDDFT), especially with PBE0 and CAM-B3LYP functionals, suggest that excited-state potential energy surface crossings lead to the efficient and fast decomposition of carbonyl azides upon photoexcitation. Especially, the decay of the Franck–Condon state to the S1 state with π(CON3)–π*(N3′) transition character, where –N3 is in a bent conformation (∠NNN = ca. 125°), is the key step. Finally, a model is presented to explain solvent dependence, different decaying rates, and other experimental findings.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
