Asymmetric abstraction of two chemically-equivalent methylene hydrogens: significant enantioselectivity of endoperoxide presented by fumitremorgin B endoperoxidase†
Abstract
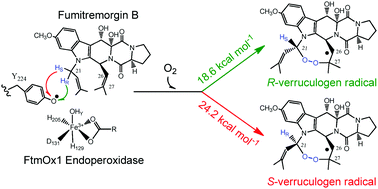
The combination of the inert C–H bond activation and asymmetric synthesis, especially the transformation of prochiral sp3 precursors to chiral sp3 centers, is a profound challenge. In the present DFT calculations, the unique enantioselectivity in verruculogen biosynthesis catalyzed by fumitremorgin B endoperoxidase (FtmOx1) has been mechanistically investigated, where a prochiral methylene in fumitremorgin B is dominantly converted to an R-chiral eight-membered endoperoxy ring. FtmOx1 is the first-reported mononuclear α-ketoglutarate-dependent non-heme iron enzyme responsible for chiral endoperoxide formation, which handles the substrate using a Tyr224 radical resulting from the hydrogen abstraction by an FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species. It is demonstrated that the perfect enantioselectivity of the R-endoperoxy ring originates from the asymmetric abstraction of two chemically-equivalent methylene hydrogens from substrate chain A by the Tyr224 radical and the high conformation stability of the resultant chain A radical due to steric effects. The barrier difference in the abstraction of two hydrogens is 5.6 kcal mol−1. The hydrogen abstraction by the Tyr224 radical is rate-limiting in the FtmOx1 reaction with an overall barrier of 18.6 kcal mol−1. The results obtained here advance the understanding of the chemistry in enantioselectivity, providing a potentially general way for the transformation of prochiral sp3 precursors to chiral sp3 centers.
O species. It is demonstrated that the perfect enantioselectivity of the R-endoperoxy ring originates from the asymmetric abstraction of two chemically-equivalent methylene hydrogens from substrate chain A by the Tyr224 radical and the high conformation stability of the resultant chain A radical due to steric effects. The barrier difference in the abstraction of two hydrogens is 5.6 kcal mol−1. The hydrogen abstraction by the Tyr224 radical is rate-limiting in the FtmOx1 reaction with an overall barrier of 18.6 kcal mol−1. The results obtained here advance the understanding of the chemistry in enantioselectivity, providing a potentially general way for the transformation of prochiral sp3 precursors to chiral sp3 centers.


 Please wait while we load your content...
Please wait while we load your content...