An efficient cluster model to describe the oxygen reduction reaction activity of metal catalysts: a combined theoretical and experimental study†
Abstract
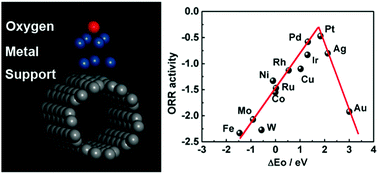
A simple and efficient cluster model containing only seven metal atoms was proposed to investigate the oxygen reduction reaction (ORR) activity of various metal catalysts by density functional theory (DFT) calculation. The model was validated by comparing ORR volcano plots obtained from the cluster model in this work and the slab model in the literature. We then used this model to investigate the influence of the support of Ag nanoparticles on ORR activity, which is hard to describe by the slab model. The calculations revealed the binding energy of oxygen atoms on Ag/COOH-CNTs or Ag/OH-CNTs changed to 2.04 and 2.09 eV respectively, in comparison to that of Ag/CNTs (2.13 eV). As a result, the ORR current density improved to 2.24 and 1.88 mA cm−2 at the potential of 0.7 V (vs. RHE) for Ag/COOH-CNTs and Ag/OH-CNTs respectively, in comparison to that of Ag/CNTs (1.66 mA cm−2). The cluster model could simultaneously reduce the computing time and make it possible to consider the influence of catalyst supports, which would provide new insight to design more effective ORR metal catalysts.


 Please wait while we load your content...
Please wait while we load your content...