Elucidation of the intrinsic optical properties of hydrogen-bonded and protonated flavin chromophores by photodissociation action spectroscopy†
Abstract
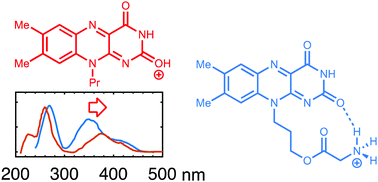
A model system of the flavin chromophore was synthesized and investigated for its intrinsic optical properties by gas phase action spectroscopy using an ion storage ring. An ammonium group was anchored to this flavin chromophore to allow its transfer to the gas phase by electrospray ionization and for studying the influence of hydrogen bonding and a nearby positive charge. According to calculations one of the hydrogen atoms of the ammonium group favorably forms an intramolecular ionic hydrogen bond to one of the oxygen atoms of the flavin chromophore, and this interaction was found to cause a blueshift of the S0 → S1 transition and a redshift of the S0 → S2 transition. For comparison, the S0 → S1 transition shows little solvent dependence (only in regard to the degree of fine structure). In addition, the influence of protonation of the flavin chromophore was elucidated by experimental and theoretical studies of a simple flavin system. While the position of the S0 → S1 absorption was at identical positions in the gas phase for the intramolecularly hydrogen-bonded and protonated flavin systems, the S0 → S2 absorption was further redshifted for the protonated species. This redshift resulting from protonation was also observed in solution.


 Please wait while we load your content...
Please wait while we load your content...