Hydration in aqueous solutions of ectoine and hydroxyectoine
Abstract
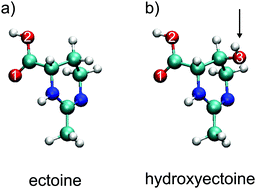
We explore the influence of the two osmolytes ectoine and hydroxyectoine on the structure of pure water and aqueous NaCl solutions using non-resonant X-ray Raman scattering spectroscopy at the oxygen K-edge. Both ectoine and hydroxyectoine are naturally occurring organic osmolytes synthesized by halophilic organisms that live in high-salt and other extreme environments. We find that X-ray spectroscopic data at the oxygen K-edge are consistent with a scenario where both osmolytes affect the hydrogen bonding network of water on a local scale to in effect increase tetrahedral order. This supports the proposed stabilizing mechanism of the osmolytes for proteins: preferential exclusion of the osmolytes from the proteins' surface and preferential hydration of the macromolecules instead of complex alterations to the structure of water on a bulk scale. The effect of NaCl on water, a disruption of hydrogen bonds and tetrahedral order, acts in opposition to the localized water-binding effects of ectoine and hydroxyectoine. For ternary mixtures of osmolyte in the presence of NaCl, the effects seen in the spectra are found to be additive such that the mixed solutes generate a level of oppositional frustration in the water network.


 Please wait while we load your content...
Please wait while we load your content...