The evaporation kinetics of pure water droplets at varying drying rates and the use of evaporation rates to infer the gas phase relative humidity
Abstract
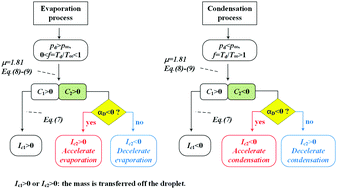
Numerous analytical models have been applied to describe the evaporation/condensation kinetics of volatile components from aerosol particles for use in many applications. However, the applicability of these models for treating cases that lead to substantial and rapid changes in particle temperature due to, for example, evaporative cooling remain to be compared with measurements. We consider three typical treatments, comparing predictions of the evaporation rates of pure water droplets over a wide range in gas phase relative humidity (RH) and exploring the sensitivity of the predictions to uncertainties in the thermophysical gas and condensed-phase parameters. We also compare predictions from the three treatments to measurements of the evaporation rates of pure water droplets with varying RH using an electrodynamic balance (EDB), concluding that only two of the model treatments are sufficiently able to account for the level of evaporative cooling (typically as high as 12 K). Finally, we show that the RH can be inferred accurately from the evaporation rate of pure water droplets over the full range in accessible RH and comparison with the model predictions (within absolute uncertainties of 2.5% RH over the range 20% to 95% RH), considering the level of agreement with independent measurements made through determining the equilibrated size of aqueous sodium chloride and sodium nitrate droplets.


 Please wait while we load your content...
Please wait while we load your content...