Theoretical rationalisation of the photophysics of a TICT excited state of cinnamoyl–coumarin derivatives in homogeneous and biological membrane models†
Abstract
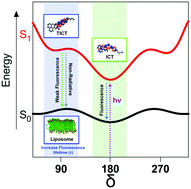
A new hybrid cinnamoyl–coumarin probe was synthesised to study the formation and dynamics of a twisted internal charge transfer (TICT) excited state in homogeneous and biological membrane models. This probe showed a large bathochromic shift of the fluorescence band with the solvent polarity, which is associated with the decrease in the fluorescence intensity due to fast non-radiative deactivation pathways, ascribed to TICT excited state formation in polar solvents. The calculated potential energy surfaces using density functional theory (DFT) and time dependent-DFT (TD-DFT) along with the energetic barriers calculated using the ABF methodology established the energy requirements for a rotational twisting of the cinnamoyl–coumarin bond for TICT excited state formation. This strategy has allowed estimating the role of the ground state conformation and excited state distribution that, concomitant with fluorescence lifetime measurements, describes in detail dual fluorescence emission from TICT and ICT excited states. Moreover, the high sensitivity of fluorescence lifetimes of the TICT excited state in liposomes allows us to propose the use of this type of probes as a powerful tool for the study of gel and crystalline liquid phases in lipid membrane models. The development of this new approach will allow rationalizing and understanding the photochemical behavior of fluorescent TICT-based probes in constrained biological environments.


 Please wait while we load your content...
Please wait while we load your content...