Triplet electron transfer and spin polarization in a palladium porphyrin–fullerene conjugate
Abstract
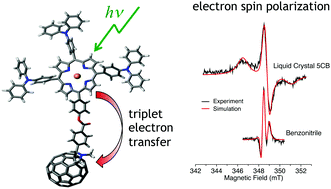
Transient electron paramagnetic resonance (TREPR) spectroscopy is used to investigate the pathway and dynamics of electron transfer in a palladium porphyrin–fullerene donor–acceptor conjugate. The heavy Pd atom in the porphyrin greatly enhances the rate of intersystem crossing and as a result, electron transfer from the porphyrin to fullerene occurs via the porphyrin triplet state. The sign of the polarization pattern of the radical pair generated by the electron transfer is opposite in benzonitrile and the liquid crystal 5CB. This difference is the result of a change in sign of the spin–spin coupling, which allows the values of the dipolar and exchange couplings between the electrons in the charge-separated state to be estimated. In addition to the radical pair, signals from the fullerene triplet state are also observed. The polarization of the fullerene triplet state inverts with time, while the radical pair signal decays to a multiplet pattern that persists for times longer than the spin–lattice relaxation time. A kinetic model, developed to explain these effects, reveals that forward and reverse electron transfer between the charge-separated state and the fullerene takes place. This process, combined with singlet recombination of the radical pair accounts for the inversion of the fullerene triplet state polarization and the long-lived multiplet polarization of the radical pair.


 Please wait while we load your content...
Please wait while we load your content...
