Revealing the role of phosphoric acid in all-vanadium redox flow batteries with DFT calculations and in situ analysis†
Abstract
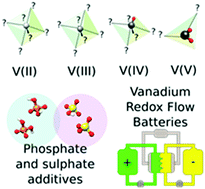
The present work suggests the use of a mixed water-based electrolyte containing sulfuric and phosphoric acid for both negative and positive electrolytes of a vanadium redox flow battery. Computational and experimental investigations reveal insights on the possible interactions between the vanadium ions in all oxidation states and sulphate, bisulphate, dihydrogen phosphate ions and phosphoric acid. In situ cycling experiments and ion-specific electrochemical impedance measurements confirmed a significant lowering of the charge-transfer resistance for the reduction of V(III) ions and, consequently, an increase of the voltaic efficiency associated with the negative side of the battery. This increase of performance is attributable to the complexation of this oxidation state by phosphoric acid. So far, mixed acids have mainly been discussed with the focus on V(V) solubility. In this work we rationalize the impact of the mixed acids on the electrochemical efficiency opening new strategies on how to improve the cycling performance with ionic additives.


 Please wait while we load your content...
Please wait while we load your content...