A unique metallic phase of H3S at high-pressure: sulfur in three different local environments†
Abstract
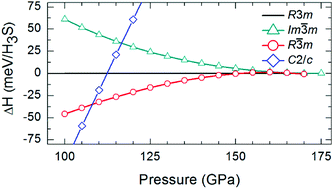
We propose a new metallic phase (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, Z = 24) for H3S in a large pressure interval (∼108–166 GPa) using an evolutionary crystal structure search algorithm combined with first-principles calculations. This structure consists of SH6, SH3 and S units which are connected through strong S⋯H hydrogen-bonds. It supports four-types of S⋯H hydrogen-bonds that symmetrize at ∼166 GPa. This is the only phase in the H–S family where sulfur exists in three different local environments. Sulfur in SH6 behaves as a cation, like in the SF6 molecule, whereas other sulfurs behave as anions. Hydrogens in SH6 behave like halogens (anions) whereas other hydrogens behave like alkalis (cations). The new structure has a substantially smaller DOS at the Fermi-level in comparison to earlier structures R3m and Im
m, Z = 24) for H3S in a large pressure interval (∼108–166 GPa) using an evolutionary crystal structure search algorithm combined with first-principles calculations. This structure consists of SH6, SH3 and S units which are connected through strong S⋯H hydrogen-bonds. It supports four-types of S⋯H hydrogen-bonds that symmetrize at ∼166 GPa. This is the only phase in the H–S family where sulfur exists in three different local environments. Sulfur in SH6 behaves as a cation, like in the SF6 molecule, whereas other sulfurs behave as anions. Hydrogens in SH6 behave like halogens (anions) whereas other hydrogens behave like alkalis (cations). The new structure has a substantially smaller DOS at the Fermi-level in comparison to earlier structures R3m and Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. This implies a lower superconducting temperature (TC) for the new phase and thus questions the validity of earlier high-TC explanations. We also find that S–H covalent bonds are different from the B–B bonds of MgB2.
m. This implies a lower superconducting temperature (TC) for the new phase and thus questions the validity of earlier high-TC explanations. We also find that S–H covalent bonds are different from the B–B bonds of MgB2.


 Please wait while we load your content...
Please wait while we load your content...