Stoichiometric network analysis of entropy production in chemical reactions
Abstract
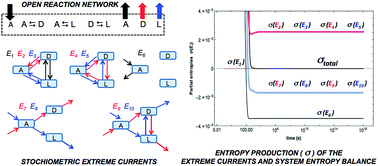
We use stoichiometric network analysis (SNA) to obtain a flux-based approach for the evaluation and description of the entropy production and exchange for chemical reactions in open systems. For non-equilibrium stationary states (NESS) the production and exchange are expressed as functions over the convex cone of the stationary reaction rates, revealing the reaction pathways and elementary flux modes (EFM) responsible for both entropy production and balance. The analysis of the entropy production of EFMs leads to a unique description of the contribution of the coupling between linear or cyclic reaction network paths, and the fluxes due to the matter exchange of the system with the system's surroundings. Network stoichiometry leads to an independent proof and confirmation of Prigogine's theorem of minimum entropy production for the linear regime of non-equilibrium thermodynamics. Moreover, the non-linear thermodynamic regime allows us to test the validity of the General Evolution Criterion (GEC) for NESS in isothermal chemical networks in mechanical equilibrium.


 Please wait while we load your content...
Please wait while we load your content...