Stability of hydrolytic arsenic species in aqueous solutions: As3+vs. As5+
Abstract
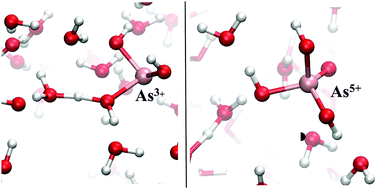
Notwithstanding the fact that arsenic compounds are ubiquitous in the As3+ and As5+ forms in aqueous solutions, most of the microscopic features underlying the conditions of the hydrolysis steps are completely unknown. This way, a first-principles description of the fundamental behaviour of common arsenic species in natural waters and biological fluids is still lacking. Here we report on a synergistic computational and experimental investigation on As3+ and As5+ speciation in aqueous solution under both standard and sizably different alkaline circumstances. If, on the one hand, ab initio molecular dynamics simulations have been used to microscopically trace the different hydrolysis steps of As3+ and As5+ by explicitly taking into account the solvent contribution, on the other hand, they have been able to identify – and predict – the most stable hydrolytic species. In addition, by means of potentiometric and calorimetric measurements, the thermodynamic parameters (log K, ΔH, and TΔS) have been determined at different ionic strength values (0 < I ≤ 1 mol L−1). By comparing the computational and the experimental findings of the species distribution under conditions of some biological fluids, a qualitative agreement on the compounds formed by As3+ and As5+ is thoroughly recorded and, therefore, the stable hydrolytic arsenic species present in natural waters and other biosystems are fully characterised.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...