Reaction kinetics of OH + HNO3 under conditions relevant to the upper troposphere/lower stratosphere†
Abstract
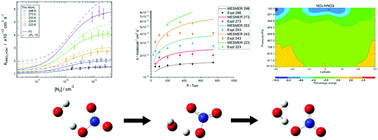
The OH initiated oxidation of HNO3 in the UT/LS plays an important role in controlling the O3 budget, removing HOx radicals whilst driving NOx/y partitioning chemistry by yielding NO3 radicals: OH + HNO3 → H2O + NO3. In this paper, k1(T, P) was measured using OH (A ← X) Laser Induced Fluorescence (LIF) and the data was modelled over the 223–298 K temperature and 25–750 Torr pressure ranges, using the modified Lindemann–Hinshelwood expression 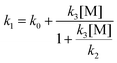 , where k0 = 5.2 × 10−14 exp(200/T) cm3 s−1, k2 = 8.4 × 10−17 exp(1900/T) cm3 s−1 and k3 = 1.6 × 10−34 exp(1745/T) cm3 s−1. A significant source of experimental uncertainty derives from accurate determination of HNO3 concentration, which is impacted by heterogeneous uptake of the low volatility HNO3 onto cold surfaces of the reactors. Our results represent the determination of k1(T, P) using two different in situ [HNO3] measurements: VUV absorption and a new two photon Photolysis Induced Fluoresence (PIF). Experimental results are discussed along with a computational master equation calculation (MESMER), which highlight the need for further theoretical study into the OH + HNO3 mechanism and potential energy surface. The atmospheric impact of these new rate constants were modelled using the STOCHEM-CRI chemistry transport global model, which have shown a small reduction in global budgets of key atmospheric species, with more significant changes in the NOx/HNO3 ratio, peaking in the tropical upper troposphere regions.
, where k0 = 5.2 × 10−14 exp(200/T) cm3 s−1, k2 = 8.4 × 10−17 exp(1900/T) cm3 s−1 and k3 = 1.6 × 10−34 exp(1745/T) cm3 s−1. A significant source of experimental uncertainty derives from accurate determination of HNO3 concentration, which is impacted by heterogeneous uptake of the low volatility HNO3 onto cold surfaces of the reactors. Our results represent the determination of k1(T, P) using two different in situ [HNO3] measurements: VUV absorption and a new two photon Photolysis Induced Fluoresence (PIF). Experimental results are discussed along with a computational master equation calculation (MESMER), which highlight the need for further theoretical study into the OH + HNO3 mechanism and potential energy surface. The atmospheric impact of these new rate constants were modelled using the STOCHEM-CRI chemistry transport global model, which have shown a small reduction in global budgets of key atmospheric species, with more significant changes in the NOx/HNO3 ratio, peaking in the tropical upper troposphere regions.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
