Structural, vibrational and electronic properties of the superconductor CuxTiSe2: theoretical and experimental insights
Abstract
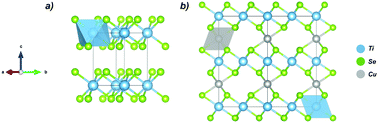
The crystal/electronic structure and vibrational properties of the CuxTiSe2 intercalation compounds were studied combining experimental and theoretical techniques. The Cu added into the TiSe2 matrix was characterized as an intercalant atom into van der Waals gaps from Raman spectroscopy analysis. Theoretical and experimental data indicate the Cu-intercalation effect on the crystalline structure as a local disorder affecting [TiSe6] clusters from Se⋯Se layers, which results in a volume expansion. A significant charge transfer from Cu atoms to the host lattice results in a change from Ti4+ to Ti3+ species, narrowing the band-gap and increasing the superconductivity of the material.


 Please wait while we load your content...
Please wait while we load your content...