Entropy in multiple equilibria, compounds with different sites†
Abstract
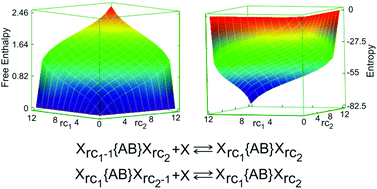
The influence of entropy in multiple chemical equilibria is investigated for systems with different types of sites for the condition that the binding enthalpy of the species is the same within each type of sites and independent of those species that are already bonded. This allows splitting of the free reaction enthalpy into the particle distribution term and all other contributions for each type of sites separately and, hence, to evaluate this entropy contribution to the free reaction enthalpy. The situations for which this applies can be chemically very different, e.g. acid base, ligand exchange, isomerisation, conformational change, rearrangement of a ligand, ion exchange, adsorption of a species on the surface of a particle or a dendrimer, insertion of charged or neutral species into the cavities of a microporous or mesoporous host. We provide physical insight by discussing Xrc1{n1ABn2}Xrc2 systems. The number of coordination sites A and B are n1 and n2, respectively. The indices rc1 = 1, 2,…,n1 and rc2 = 1, 2,…,n2 count the number of X bonded to sites A and sites B, respectively. An important result is that the large number of equilibrium constants needed to describe those situations can be expressed as a function of two constants only. This allows studying systems quantitatively by experimental and theoretical means which otherwise might be difficult to handle. It has also implication for theoretical studies in the sense that it is sufficient to model only two reactions instead of many in order to describe a system. The results remain valid for systems with more than two types of different sites. The description of the entropy driven development of the fractional equilibrium coverage of the sites provides a new tool for understanding adsorption and ion exchange isotherms. The fractional equilibrium coverage of the sites can be described as a linear combination of individual Langmuir isotherms despite of the fact that such a linear combination has never the shape of the original Langmuir isotherm. This is remarkable and very useful. It provides us with new tools for describing and testing isotherms based on well defined, transparent physical ideas. Explicit solution for systems with 2, 3, 4, 5, 6, and 12 coordination sites are reported. Applications to a system with 12 coordination sites serve to illustrate information that can be obtained for complex situations.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...