The electrochemical properties of Co3O4 as a lithium-ion battery electrode: a first-principles study
Abstract
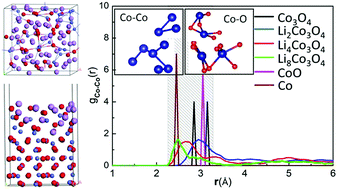
Extensive first principles calculations were performed to study the structural and electrochemical features of Co3O4 during its lithiation process as an anode material for lithium-ion batteries (LIBs). We found that with up to 8 mol Li in Co3O4, the formed LinCo3O4 structures are stable for low Li concentrations of n ≤ 1, but obvious structure distortions and volume expansions occur for LinCo3O4 with n > 1. This may be the reason why Co3O4 has a high Li capability but low cycling life as a LIB anode. The ab initio molecular dynamics simulations for LinCo3O4 (n = 2, 4, 8) further suggest a two-step electrochemistry process of Co3O4 → CoO → Co upon the lithiation process. We detected a distorted surface structure as Li atoms react with the Co3O4(110) surface, which also reduces the rate capability of the Co3O4 anode.


 Please wait while we load your content...
Please wait while we load your content...