An accurate full-dimensional potential energy surface and quasiclassical trajectory dynamics of the H + H2O2 two-channel reaction†
Abstract
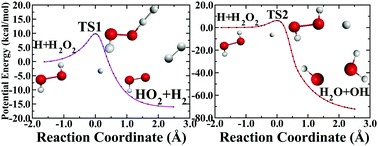
We report a new full-dimensional potential energy surface (PES) of the H + H2O2 reaction, covering both H2 + HO2 and OH + H2O product channels. The PES was constructed using the recently proposed fundamental invariant neural network (FI-NN) approach based on roughly 110 000 ab initio energy points by high level UCCSD(T)-F12/aug-cc-pVTZ calculations. The small fitting error (5.7 meV) and various tests imply a faithful representation of the discrete ab initio data over a large configuration space. Extensive quasiclassical trajectory (QCT) calculations were carried out on the new PES at a collision energy (Ec) of 15.0 kcal mol−1. The reaction yields dominantly OH + H2O, because of the lower reaction barrier and much larger reaction exothermicity (∼71 kcal mol−1) for this channel. Due to the exit barrier of both reaction channels, the most available energy is partitioned into the translational motion of the products. Considerable vibrational excitations of the H2O product are seen, particularly for the symmetric stretching and bending modes. The angular distributions show predominantly backward scattering, which is consistent with the direct rebound mechanism.


 Please wait while we load your content...
Please wait while we load your content...