Density-functional description of alkalides: introducing the alkalide state†
Abstract
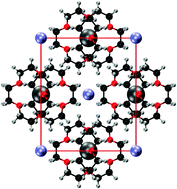
Alkalides are crystalline salts in which the anion is a negatively charged alkali metal. A systematic investigation of the electronic structure of thirteen alkalides, with known crystal structures, is conducted using density-functional theory. For each alkalide, a high-lying valence state is identified that is localised on the alkali anions and is consistent with the low band gap and strong reducing power characteristic of these materials. This ‘alkalide state’ is compared to a similar state in the related class of electride materials, where the alkali anions are replaced by crystal voids occupied by localised, interstitial electrons. Finally, a thermodynamic cycle is constructed to examine the energy differences between the alkalides and electrides, revealing that the alkali-metal anion significantly stabilises the crystal.


 Please wait while we load your content...
Please wait while we load your content...