Probing acid sites in solid catalysts with pyridine UV-Vis spectroscopy†
Abstract
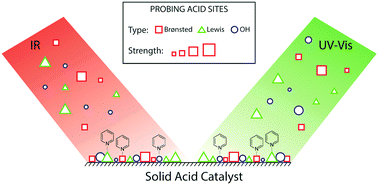
Solid acids hold widespread applications in the field of heterogeneous catalysis. In this work, we present pyridine UV-Vis spectroscopy as a novel and promising tool to study the acidic properties of such solid acids. It was found that upon interaction with acid sites, the electronic properties of pyridine change significantly. Monitoring of consecutive adsorption and desorption of pyridine revealed absorption bands in the UV-Vis region characteristic for (a) pyridinium ions formed on Brønsted acid sites, (b) pyridine coordinated to Lewis acid sites, (c) pyridine hydrogen-bonded to surface hydroxyl groups, and (d) physisorbed pyridine. The classical pyridine FT-IR method probes the presence of different Brønsted and Lewis acid sites as well, but lacks sensitivity towards the differentiation between surface hydroxyl groups. In contrast, the pyridine UV-Vis spectroscopy method proves especially useful for the identification and distinction of different surface hydroxyl groups, since the band position in the UV-Vis spectrum strongly depends on the chemical environment of the hydroxyl group. Moreover, utilizing a slow desorption procedure under N2 flow, it was possible to study the differences in acidic strength of the hydroxyl groups. This method and related measurement protocols were developed for the study of acidic properties within solid acids with different silica/alumina ratios, but are, in our opinion, more generally applicable to any solid acid.


 Please wait while we load your content...
Please wait while we load your content...