Regium bonds between Mn clusters (M = Cu, Ag, Au and n = 2–6) and nucleophiles NH3 and HCN†
Abstract
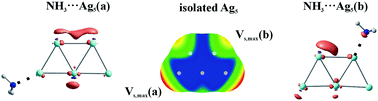
The most stable geometries of the coinage metal (or regium) atom (Cu, Ag, Au) clusters Mn for n up to 6 are all planar, and adopt the lowest possible spin multiplicity. Clusters with even numbers of M atoms are thus singlets, while those with odd n are open-shell doublets. Examination of the molecular electrostatic potential (MEP) of each cluster provides strong indications of the most likely site of attack by an approaching nucleophile, generally one of two positions. A nucleophile (NH3 or HCN) most favorably approaches one particular M atom of each cluster, rather than a bond midpoint or face. In the closed-shell clusters, the interaction energies are highly dependent upon the intensity of the MEP, but this correlation fades for the open-shell systems studied in this work. The strength of the interaction is also closely related to the basicity of the nucleophile. Regium bond energies can be more than 30 kcal mol−1 and tend to follow the Au > Cu > Ag order. These interaction energies are in large part derived from Coulombic attraction, with a smaller orbital interaction contribution.
- This article is part of the themed collections: 1st International Conference on Noncovalent Interactions and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...